This videos shows how to draw the lewis structure of gai3 gallium triiodide. Therefore it has 5 electrons in its outermost shell.
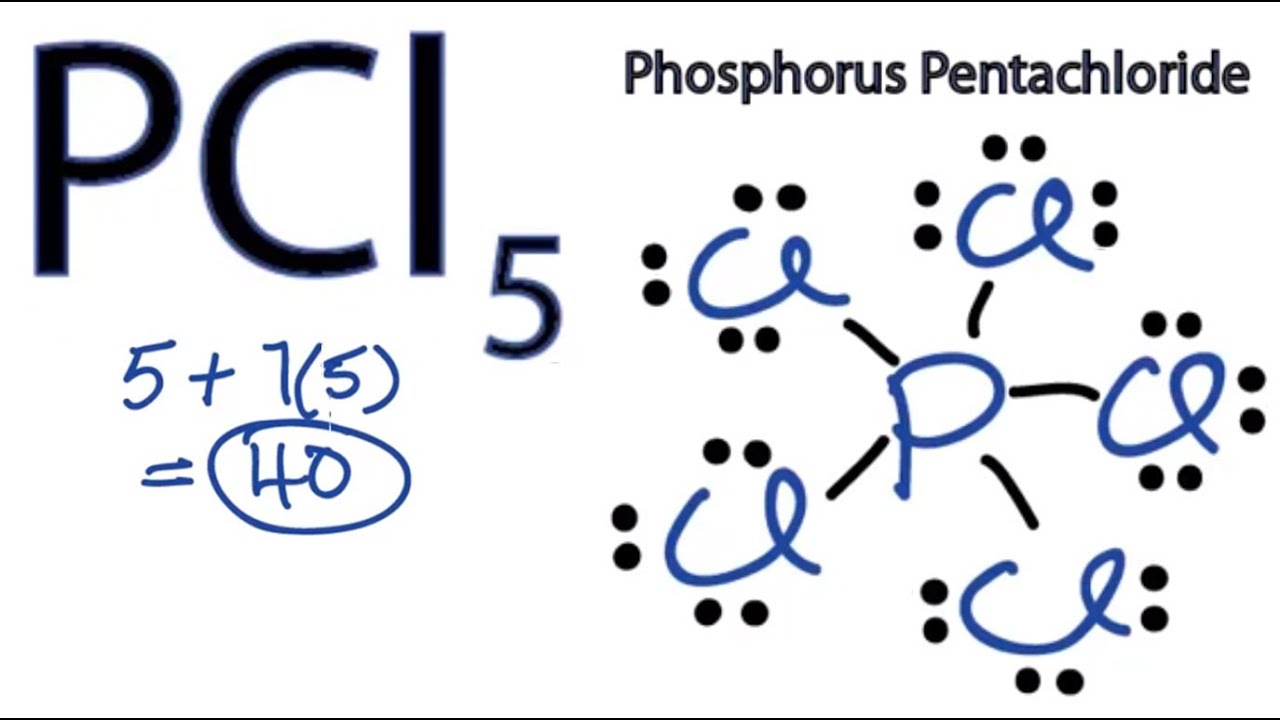
Pcl5 Lewis Structure How To Draw The Lewis Structure For Pcl5 Youtube
Choose a Central Atom.
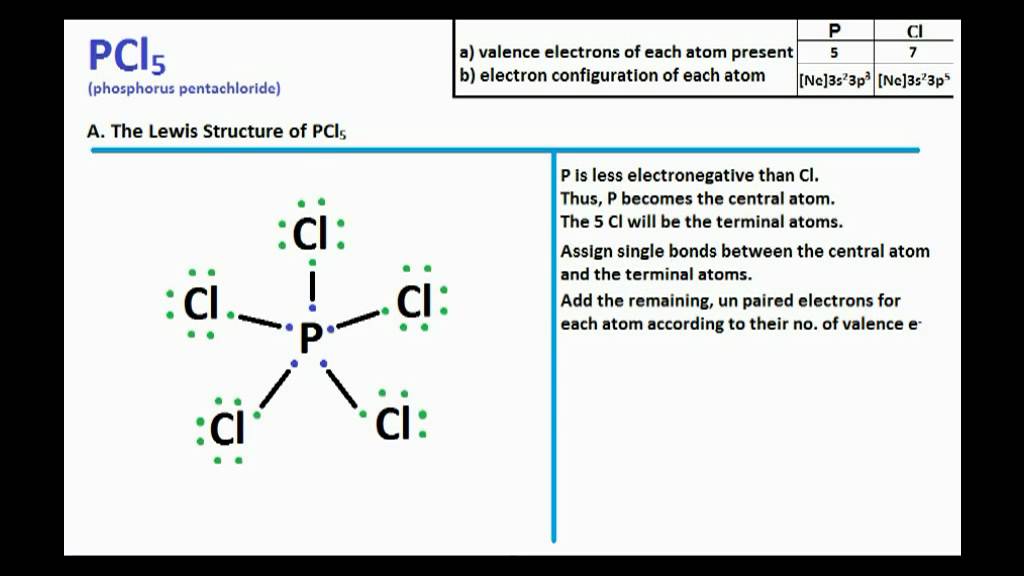
. There are several steps to draw the lewis structure of O 3. Drawing the Lewis structure of PCl5. The lewis structure of n2h2 shows.
N 2 h 2 is straightforward with no double or triple bonds. The octet rule can be satisfied for all non-hydrogen atoms when the remaining unpaired electrons are moved in to form a double bond between carbon and oxygen. Given a chemical formula.
See the answer See the answer done loading. There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. This chemistry video tutorial provides a basic introduction into drawing lewis dot structures but most importantly it provides an explanation on how to calc.
Also lone pair electrons are also called unshared electrons silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it. What is the bond angle around each carbon center. See the answer See the answer done loading.
You can see there is no lone pairs on phosphorus atom in PCl 5 as PCl 3. Determine the Number of Bonds in the Molecule. Total electron pairs are determined by dividing the number total valence electrons by two.
Find the Total Number of Valence Electrons. Total valence electrons pairs. Ni3 ph3 pf3 pcl3 pbr3 pi3.
For N 3- ion total pairs of electrons are 8. Also the electron geometry of PCl3 is tetrahedral. Count the number of valence electrons in a PCl5 molecule.
This problem has been solved. If you do not have a gradual hand however there are lots of nail stickers stamps or head on to your neighborhood nail salon so they can replicate it for you. Find the Number of Electrons Needed to Make the Atoms Happy Step 3.
Now lets see the proper steps to draw a lewis structure-1. A Lewis structure is a graphic representation of the electron distribution around atoms. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step.
But because ammonia is a simple molecule these steps are not complex and do not. When you follow these steps properly you can learn how to. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis structure for the molecule.
Rules for drawing Lewis structures. How to Draw a Lewis Structure. Steps of drawing PCl 3 lewis structure.
Phosphorus having atomic number 15 has an electron composition of 2 8 5. Another one has two lone pairs and remaining one has three lone pairs. A Lewis structure also helps to make a prediction about the geometry of a molecule.
Find total number of electrons of the valance shells of phosphorus and chlorine atom. Who are the experts. There are guidelines to draw lewis structures.
The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. In the lewis structure for n2h2 there are a total of 12 valence electrons. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells.
In this tutorial we will learn how to draw the lewis structure of PCl 5 step by step with all theories. Since the ph3 has a lone pair out of three bond pairs its geometrical shape will be. There are a total of 40 valence electrons in the PCl5 Lewis structure.
You have to follow several steps to draw the lewis structure of NH 3. It is a volatile liquid that reacts with water and releases HCl gas. Include lone pairs NCl3.
In the lewis structure of NH 3 there are three N-H bonds and one lone pair on nitrogen atom. Now we are going to study each step of drawing the lewis structure of PCl 3. Number of steps can be changed according the complexity of the molecule or ion.
Solution for Draw the Lewis structure of HCN. Draw the lewis structure of the following molecule. Place Electrons Around Outside Atoms.
Phosphorus pentachloride decomposes according to the chemical equation. Include All Lone Pairs Of Electrons. Now there is a clear difference between electron geometry and molecular.
Thus the chart clearly shows that the molecular geometry of PCl3 is a trigonal pyramid. We review their content and use your feedback to keep the quality high. Now the central atom is generally the least electronegative.
Also one oxygen atom has a -1 charge and another oxygen atom has a 1 charge. We can refer to the periodic table for this. PCl3 Molecular Electron Geometry Lewis Structure Bond Angles and Hybridization.
Hence 4 chlorine atoms 3 lone pairs on each 12 lone pairs. Now PCl3 is a AX3E type molecule where A central atom X surrounding atom E lone pair. Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds.
1 draw the lewis structure for ch3nco a neutral molecule. PCl 5 lewis structure. In PCl 5 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond sigma bond.
Molekülün lewis nokta yapısı yazılır. One bonded pair contains two electrons hence 4 2 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. PCl5 g PCl3 gCl2 g Kc 180 at 250 degrees Celsius A 0352 mol sample of PCl5 g is injected into an empty 445 L reaction vessel held at 250 C.
Steps of drawing lewis structure of NH 3. We come to understand that PCl5 is made up of Phosphorous and Chlorine. Hydrogen h only needs two valence electrons to have a full outer shell.
The molecule of ozone has three oxygen atoms. From three oxygen atoms one oxygen atom has one lone pair. PCl3 has 1 lone pair and 3 surrounding atoms.
The image shows the Lewis dot structure of isoflurane. This means that it can hold more than 8 valence electrons. Drawing bonds in place of pairs of electrons composed of one electron from each atom yields a bonded skeletal structure.
Steps of drawing lewis structure of O 3. Experts are tested by Chegg as specialists in their subject area. Total electrons pairs existing as lone pairs.
07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3. Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table. Draw the Lewis Structure for PCl 5.
Calculate the concentrations of. Isoflurane is used as an inhaled anesthetic. Draw a Skeletal Structure.
Draw the Lewis structure of the following molecule. Draw the lewis structure for pcl5 include lone pairs Youll need a gradual hand for this eye catching nail design but it surelys oh-so worthwhile.

Pcl5 Lewis Structure And Molecular Geometry Youtube

Draw The Lewis Structure For Pcl5 And Answer The Following Questions A Does The Central Atom Violate The Octet Rule B How Many Lone Pairs Of Electrons Are In The Molecule C
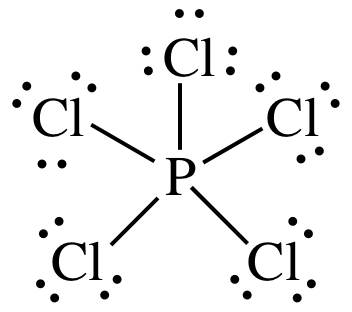
Chapter 11 Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory
Lewis Structure Of Pcl5 Biochemhelp
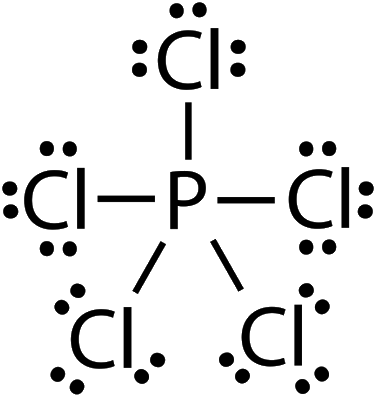
Why Pcl5 Is Polar Or Nonpolar The Chemical Compound Pcl5 Is Also By Anisha Verma Medium
What Is The Lewis Structure Of Pcl5 Quora
0 comments
Post a Comment